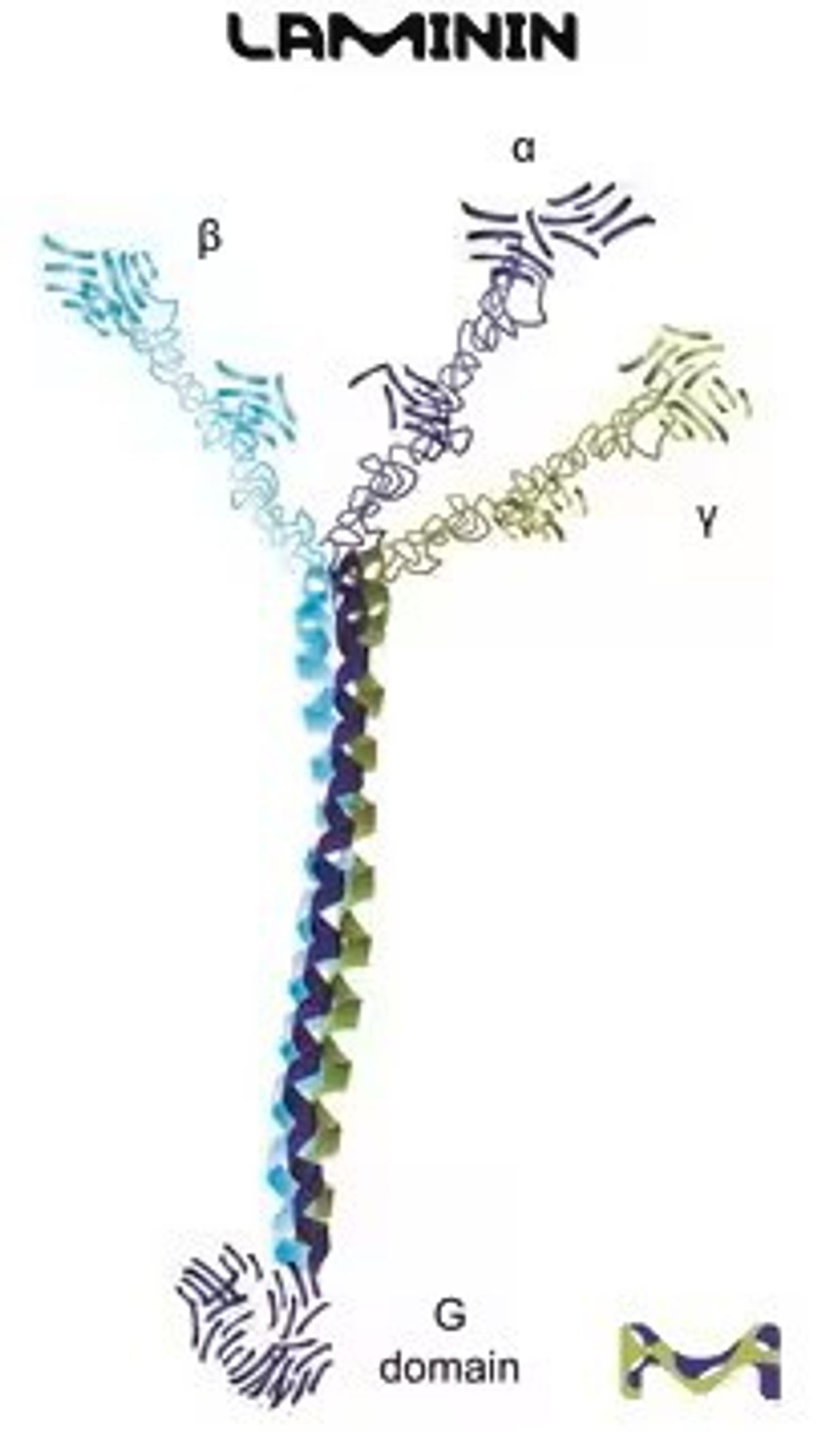
The numerous cells that make up mammalian tissues are surrounded by an intricate network of proteins known as the extracellular matrix (ECM). Together, these proteins provide cells with a structural scaffold, enable communication between cells, and stimulate molecular signaling for a wide range of cellular activities, including proliferation, attachment, differentiation, morphology, movement, and death.
As part of broad preclinical research efforts, scientists grow and maintain mammalian cells in vitro, using them in countless ways to assess basic and translational scientific questions. Among the most essential considerations for generating reproducible and reliable cell cultures is mimicking their native ECM environment. Certain cells can do this on their own by secreting ECM proteins in vitro, but many are unable to do so. To enable viable cultures of such cells, scientists coat cell cultureware surfaces with ECM components, including naturally occurring collagen, laminin, fibronectin, gelatin, and vitronectin, as well as synthetic polymers such as poly-L-lysine and poly-L-ornithine.
The behavior of different cell types in culture is influenced by the specific ECM protein substrates and their combinations. For example, coating cultureware with laminin, poly-L-lysine, and poly-L-ornithine encourages neural stem cells to adhere and differentiate, enabling a wide range of studies.1 Other stem cell cultures, such as embryonic stem cells, benefit from ECM components found in naturally occurring gelatin—a protein mixture extracted from collagen that further enables cell attachment.2 Other ECM proteins such as vitronectin encourage differentiation and migration of various cell types, including cancer cells.3
For cell cultures that need multiple types of ECM proteins, cultureware preparation can become a tedious and lengthy process, considering that some coatings necessitate additional overnight steps and rinses are required between coatings. Moreover, with each added preparation step, the risk of contaminating cell cultures grows. In the event of contamination, scientists must dispose of the cells and restart the entire process, which consumes valuable resources and can set experiments back significantly. As a result, researchers explore novel ECM products and tools to optimize and streamline this process. For example, using pre-mixed coating solutions and pre-coated cell culture plate inserts decrease preparation time and improve reproducibility across cell cultures.
MilliporeSigma’s pre-mixed, ready-to-use ECM factors provide a significant advantage over conventional cultureware coating approaches by reducing the need for multiple applications and washing steps. Their liquid solution mixes eliminate the need for overnight incubation before adding a coating of a second attachment factor, thereby streamlining workflows and enabling same-day assays. Because the working concentrations of these ECM components are already optimized and validated, researchers avoid dilution steps and save additional time. Moreover, MilliporeSigma’s Millicoat® cell adhesion strips are pre-coated with ECM proteins and specially designed as removable components for cell culture plate frames. Overall, these solutions help researchers take the hassle out of multi-step cell culture attachment preparations and streamline lengthy workflows, saving resources and time, and improving the overall reliability of cell culture research data.
References
- Ahmed S. The culture of neural stem cells. J Cell Biochem. 2009;106(1):1-6.
- Kitajima H, Niwa H. Clonal expansion of human pluripotent stem cells on gelatin-coated surface. Biochem Biophys Res Commun. 2010;396(4):933-938.
- Schneider G, et al. Evidence that vitronectin is a potent migration-enhancing factor for cancer cells chaperoned by fibrinogen: a novel view of the metastasis of cancer cells to low-fibrinogen lymphatics and body cavities. Oncotarget. 2016;7(43):69829-69843.