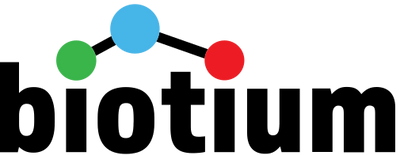
ABOVE: A human cholangiocyte–derived organoid with nuclei in blue and the cytoplasm of bile duct cells in green
FOTIOS SAMPAZIOTIS, TERESA BREVINI
Scientists have shown over the past decade or so that organoids—small, organ-like structures grown in culture from stem cells—can integrate into many organs, including the liver, lungs, and guts of mice, and repair defects. In a study published today (February 18) in Science, researchers have advanced this approach in human tissue, and demonstrate that organoids derived from adult cholangiocytes, the cells that line the bile ducts, can integrate into human livers from deceased organ donors. The findings pave the way for new treatments for liver diseases, as well as for the repair of donated organs to make more available for transplant.
“It is quite spectacular if you can really functionally repair the liver by injecting cholangiocytes into an intact liver,” says Hans Clevers, a developmental biologist at Utrecht University in the Netherlands. He was not involved in the work, but in research led by former postdoc Meritxell Huch, his group showed in 2015 that it was possible to grow human liver organoids in culture and that they could be successfully transplanted into mice—work the authors of the new study have built upon.
As a hepatologist and researcher at Cambridge University Hospitals and the University of Cambridge in the UK, Fotios Sampaziotis sees lots of patients with disorders of the bile ducts. This network of tubes runs through the liver and, when healthy, carries bile, the toxic byproduct of liver metabolism, away to the intestine where it helps digest food or to the gall bladder for storage.
In the disease known as primary sclerosing cholangitis, the bile ducts become inflamed, then scar tissue forms, which narrows and hardens the ducts. Resulting bile build-up leads to destruction of liver tissue. There is no cure, Sampaziotis says, and this disorder and other bile duct diseases are responsible for 70 percent of liver transplants in children and about one-third of liver transplants in adults.
Sampaziotis, Cambridge stem cell biologist Ludovic Vallier, Cambridge transplant surgeon Kourosh Saeb-Parsy, and colleagues showed in 2017 that organoids derived from human cholangiocytes could make bile duct–like structures when transplanted into mice. In this new study, they first wanted to understand why these diseases tend to affect certain subsets of cells, while neighboring cells escape disease, says Sampaziotis. If physicians could make the affected cells resemble the unaffected ones, they might have a new therapeutic strategy. “The question then is, how are these cells different?” he adds.
This is the great advancement of the field in terms of cholangiocyte biology and regenerative medicine.
—Akihiro Asai, Cincinnati Children’s Hospital
The researchers generated organoids from cholangiocytes from different parts of donated human biliary tract: the intrahepatic bile ducts, which are exposed to the lowest concentrations of bile; the common bile duct, which sees a midlevel concentration; and the gallbladder, which stores and concentrates bile. Then they used single-cell sequencing to examine gene expression.
The greater exposure cells had to one component of bile, known as bile acids, the higher their expression of genes encoding proteins that would protect them from degradation. When the team exposed cholangiocyte organoids to bile acids, the cells increased their expression of protective genes. This happened no matter where the cells originally came from in the liver, indicating that their gene expression is flexible and driven largely by their environment.
To confirm this flexibility, the research team then transplanted human cholangiocyte organoids, derived from the gallbladder where the cells most robustly protect themselves against bile, into mice with chemically damaged bile ducts. No matter where the donor cells engrafted in the ducts, they were able to integrate with host cells, regenerate damaged tissue, and make appropriate proteins. Mice that got organoids survived, while control animals that did not died quickly, pointing to the functional integration of the cholangiocytes.

The research team then took a fresh set of these human organoids and transplanted them into three donated human livers kept viable in an artificial system that holds the organ at body temperature while pumping oxygenated blood into the arteries and removing deoxygenated blood from its veins. The researchers used these livers because routine evaluations in advance of transplant surgery revealed that the organs weren’t healthy enough to be donated to a patient. In human livers, as in the mice, the cells contributed to duct-like structures. They also demonstrated an effect on cholangiocyte function by raising low bile pH, a symptom that can predict the future development of bile duct disorders.
“This is the great advancement of the field in terms of cholangiocyte biology and regenerative medicine,” says Akihiro Asai, a pediatric hepatologist at Cincinnati Children’s Hospital who did not participate in the work. The next step, he adds, is to test the technology on human livers that are in worse shape. The authors “did do engraftment in human liver, but that was essentially a healthy liver,” Asai explains. “So the biggest question is, what’s the best way to engraft organoids into [a] sick liver?” Diseased livers, he notes, “are a very hostile environment and can be very difficult to engraft.”
“It’s really exciting work,” agrees Neil Henderson, a regeneration biologist and hepatologist at the University of Edinburgh who was not involved in the study. “Another question is, could you potentially use these biliary organoids to treat patients with biliary diseases . . . who are not at the stage of needing transplantation?” he asks. If healing damage in a person and slowing down the course of disease is possible, it could mean that fewer transplants are necessary.
Alternatives to transplants for patients with bile duct disorders would be ideal, agrees Sampaziotis. He and his colleagues have created a company to that end and are also looking into ways to increase the viability of the organs that do become available for transplant. Before surgery, physicians assess a donated liver to determine whether it is suitable for transplant. About 20 percent of the time, he says, “we find out that the bile ducts . . . are quite badly damaged and therefore we cannot use it.” If they could improve that damage with organoid engraftment, perhaps more organs would be available for patients who need them.
F. Sampaziotis et al., “Cholangiocyte organoids can repair bile ducts after transplantation in the human liver,” Science, doi:10.1126/science.aaz6964, 2021.