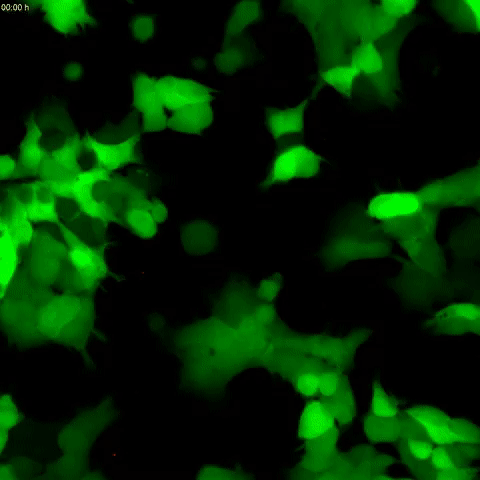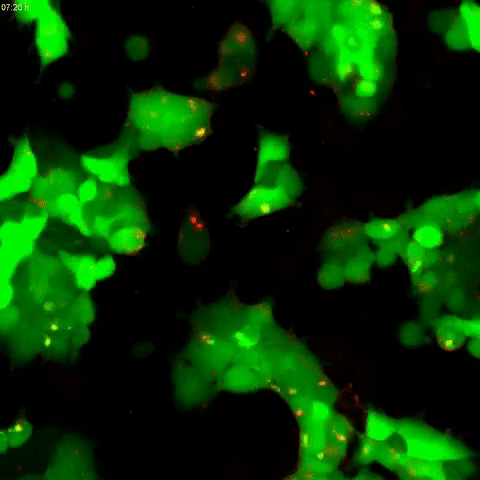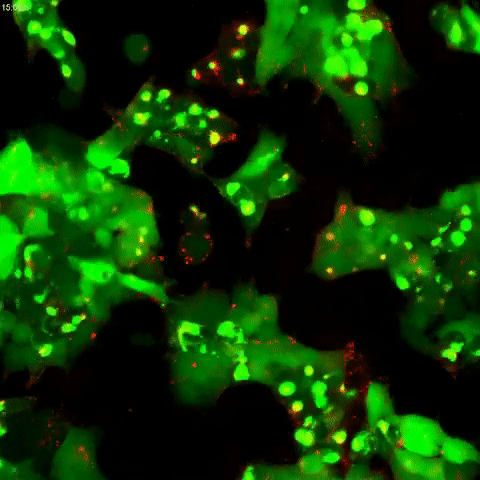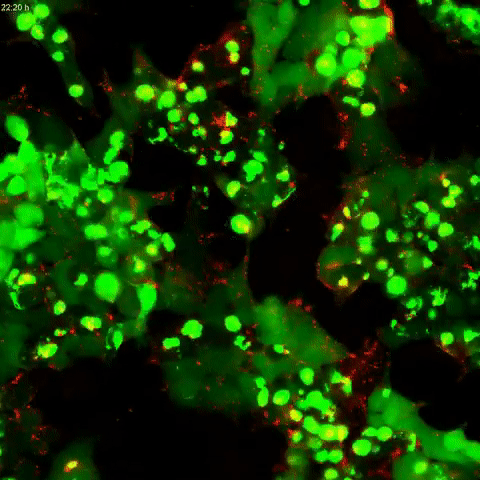
Tau protein aggregation is a mechanism behind Alzheimer’s disease and frontotemporal dementia, but the pathways controlling it have been unclear. In a study published in the Journal of Biological Chemistry on October 2, researchers found that tau aggregation is promoted by defects in endolysomes, complexes of organelles that sort and break down cell materials.
A team led by Martin Kampmann at the University of California, San Francisco, developed a human kidney cell line that had reduced expression of CHMP6, a gene responsible for endolysosomal membrane remodeling. Mutations in this gene are linked to familial frontotemporal lobar dementia and to endolysosomal defects. The researchers found that cells with reduced CHMP6 activity had “leakiness” in endolysosomal components that allowed tau seeds, or small fibrils, to escape endosomes and latch on to tau in the cell’s cytoplasm.
See “Tau Linked to RNA Splicing Errors in Flies”
J.J. Chen et al., “Compromised function of the ESCRT pathway promotes endolysosomal escape of tau seeds and propagation of tau aggregation,” J Biol Chem, doi:10.1074/jbc.RA119.009432, 2019.
Emily Makowski is an intern at The Scientist. Email her at emakowski@the-scientist.com.
Interested in reading more?




